Translate this page into:
“Treading on Thin Ice: Anesthesia Management of a Neonate with Pierre Robin Sequence for Feeding Gastrostomy and Diagnostic Bronchoscopy” – A Case Report
*Corresponding author: Dr. Amrusha Raipure, Department of Anaesthesiology and Critical Care, AIIMS, Nagpur, Maharashtra, India. dramrusha@aiimsnagpur.edu.in
-
Received: ,
Accepted: ,
How to cite this article: Sabu N, Raipure A, Balasubramanian B, Thappa P. “Treading on Thin Ice: Anesthesia Management of a Neonate with Pierre Robin Sequence for Feeding Gastrostomy and Diagnostic Bronchoscopy” – A Case Report. J Neonatal Crit Care Anesth. 2024;1:49-53. doi: 10.25259/JNCCA_11_2024
Abstract
Pierre Robin sequence (PRS) is a rare congenital anomaly characterized by the triad of micrognathia, glossoptosis, and airway obstruction, with an incidence of approximately 1 in 8500 live births. Managing neonates with PRS, particularly during anesthesia, poses significant challenges due to airway obstruction, which may be compounded by associated congenital anomalies and medical complications. This case report describes the anesthetic management of a 28-day-old female neonate with PRS and a pre-existing hypoxic-ischemic injury. The neonate presented with symptoms of recurrent regurgitation and noisy breathing, with a history of pneumonia and delayed onset sepsis, which resolved with medical management. The neonate had an orogastric tube in situ for feeding and was scheduled for a diagnostic bronchoscopy and feeding gastrostomy. On clinical examination, the neonate displayed the classical features of PRS, including micrognathia, glossoptosis, airway obstruction, and an incomplete post-palatal cleft. Imaging revealed a small patent foramen ovale with a left-to-right shunt, mild pulmonary arterial hypertension, and residual opacification in the left lower lobe, indicative of prior respiratory infection. These factors, combined with the known airway complications associated with PRS, necessitated careful anesthetic planning to ensure the safety and stability of the patient during diagnostic and therapeutic procedures. A combined general and regional anesthesia approach was selected. The neonate was induced using sevoflurane and air, and a pediatric flexible fiberoptic bronchoscope was inserted through a No. 1 i-gel as a conduit. Notably, neuromuscular blockers and opioids were avoided to maintain vocal cord mobility and minimize airway compromise. Bronchoscopy identified an adduction deformity of the posterior vocal cords. Subsequently, a gastrostomy was performed under spinal anesthesia without hemodynamic instability. In addition, a left-sided rectus sheath block was executed. Regional anesthesia minimized the reliance on systemic opioids, reduced the risks associated with general anesthesia, and facilitated a rapid recovery. Postoperatively, the neonate was transferred to the Neonatal Intensive Care Unit and demonstrated stable airway patency, normal limb movements, and adequate pain control. This case emphasizes the advantages of regional anesthesia in managing neonates with PRS. It offers a safer, opioid-sparing alternative to general anesthesia and mitigates the risks of airway compromise. We propose effective pain management techniques along with enhanced overall patient safety, thereby contributing to improved anesthetic outcomes in neonates with complex airway challenges.
Keywords
Pierre Robin sequence
Regional anesthesia
Neonatal airway management
Spinal anesthesia
Rectus sheath block
INTRODUCTION
Pierre Robin sequence (PRS) is characterized by micrognathia, glossoptosis, and airway obstruction and is usually associated with a U- or V-shaped cleft palate. The incidence is about 1 in 8500 live births equally across genders. It is considered a sequence because the various abnormalities stem from one primary developmental issue. PRS is frequently linked with other syndromes, such as Stickler, Catel–Manzke, Toriello–Carey, and Franceschetti, with 60% of cases involving additional syndromes.[1]
In addition to the above-mentioned facies complicating the neonatal airway, other associated factors such as cleft palate, history of hypoxic injury, obstructive sleep apnea, feeding difficulties, recurrent regurgitation, and pneumonia necessitate tailored anesthetic management. We present a case of a neonate with PRS planned for diagnostic bronchoscopy and feeding gastrostomy. This case underscores the advantages of regional anesthesia in managing neonates with PRS, offering a safer, opioid-sparing alternative to general anesthesia, and mitigating the risks of airway compromise while enhancing overall patient safety.
CASE REPORT
A 28-day-old female neonate, weighing 3.15 kg, was admitted with symptoms of recurrent regurgitation and noisy breathing while feeding. Her history included resolved pneumonia and late-onset sepsis, with feeding managed through an orogastric tube. Born at full term through vaginal delivery, the neonate experienced obstructive apnea and subsequent hypoxic-ischemic injury [Figure 1].
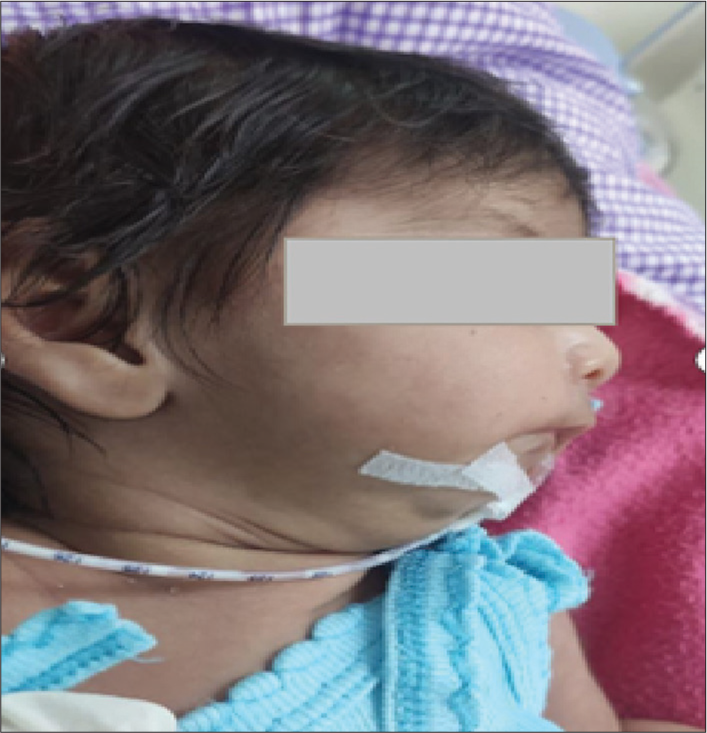
- Neonate with triad of Pierre Robin sequence.
Clinical examination revealed the classic PRS triad, including micrognathia, glossoptosis, and airway obstruction, along with an incomplete post-palatal cleft [Figure 1]. Additional findings included adventitious sounds in the left infra-axillary lung field, likely a residual effect of a previous lower respiratory tract infection. Oxygen saturation on room air was 93%. The rest of her vitals were within normal limits. Laboratory investigations were unremarkable. Imaging studies included a chest X-ray showing heterogeneous opacification in the left lower lobe, and a 2D echocardiogram revealing a small patent foramen ovale with a left-to-right shunt and mild pulmonary arterial hypertension.
Anesthetic management
These factors, combined with the known airway complications associated with PRS, necessitated careful anesthetic planning to ensure the safety and stability of the patient during diagnostic and therapeutic procedures.
The neonate was scheduled for a diagnostic bronchoscopy and feeding gastrostomy.
To address the anticipated airway management difficulties, a combined general and regional anesthesia approach was selected. The surgeons had initially planned to do a diagnostic rigid bronchoscopy. However, considering the airway handling and the anesthesia requirements for a more invasive procedure in an already complex airway, the possibility of considering a flexible bronchoscopy was discussed with the pediatric surgeons. After the surgeons were on the same page, the plan was to insert a supraglottic airway (SGA) device under a minimal inhalational anesthetic dose and perform the flexible bronchoscopy through the SGA that will act as a conduit. The neonate was induced using sevoflurane and air, and i-gel No 1 was passed. A 3.2 mm pediatric flexible fiberoptic bronchoscope was inserted through the i-gel as a conduit. Notably, neuromuscular blockers and opioids were avoided to maintain vocal cord mobility and minimize airway compromise. Bronchoscopy identified an adduction deformity of the posterior vocal cords, potentially secondary to perinatal hypoxic injury or an additional manifestation of PRS [Figure 2]. A clip is displayed illustrating the results of flexible fiberoptic bronchoscopy, revealing that the posterior part of vocal cords does not adduct despite stimulation [Video].
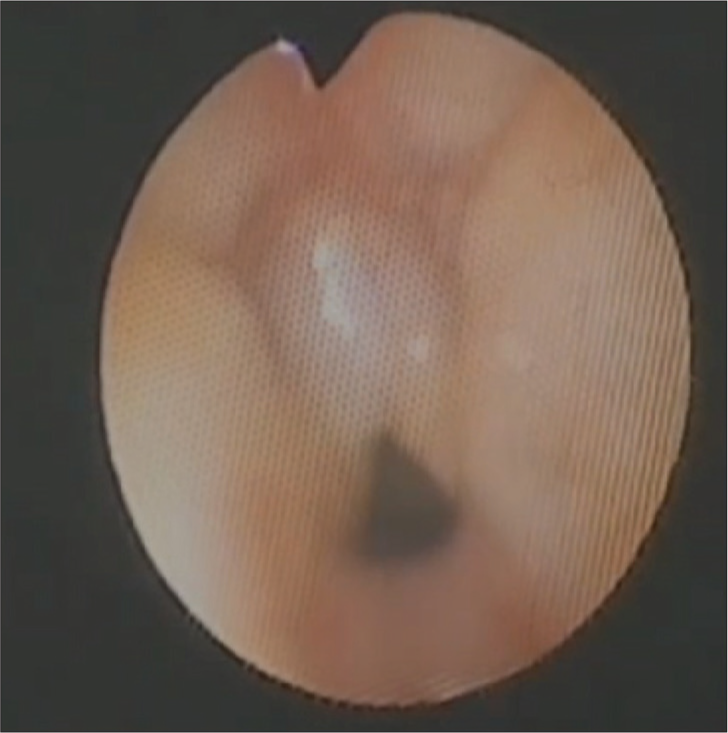
- Image from bronchoscopy showing adduction deformity of vocal cords.
It was planned to do the gastrostomy under subarachnoid block (SAB). Following bronchoscopy, the neonate was given a left lateral position for SAB. 3 mg of hyperbaric bupivacaine [0.6 mL of 0.5% bupivacaine (heavy)] was administered at the L4–L5 intervertebral space [Figure 3]. Sevoflurane was stopped after the SAB was given. The procedure, lasting approximately 1 h, was uneventful [Figure 4]. In addition, a left-sided rectus sheath block under ultrasound guidance was performed using 2.5 mg of bupivacaine and 0.4 mg of dexamethasone at the end of the procedure. It was planned to remove the SGA, once the neonate was awake and did not tolerate the SGA device. However, the neonate tolerated the SGA through the procedure breathing spontaneously, and it was removed only at the end of the surgery. The neonate successfully maintained airway patency in the postoperative period.
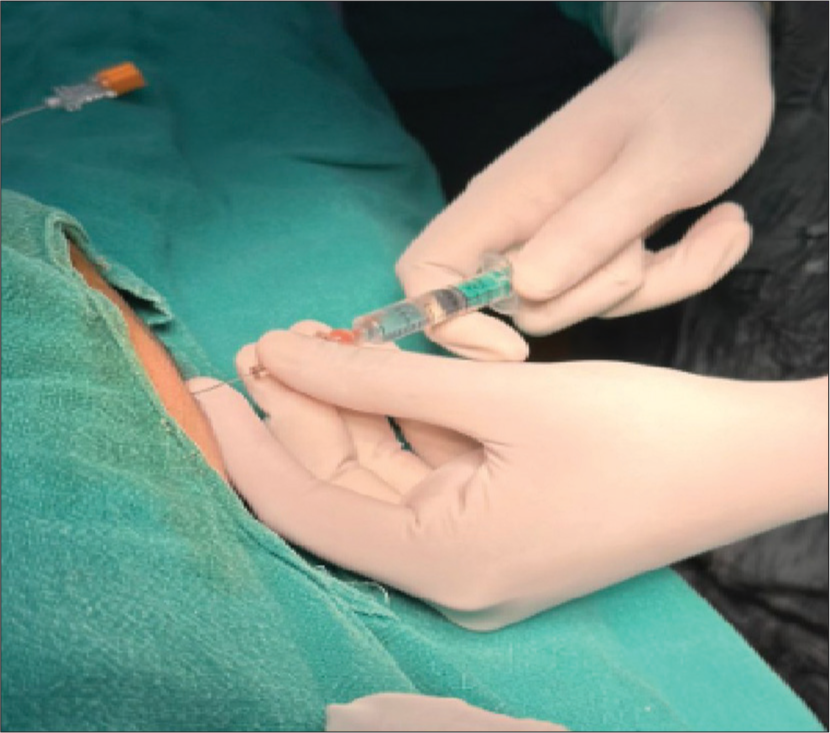
- Neonatal spinal in the left lateral position.
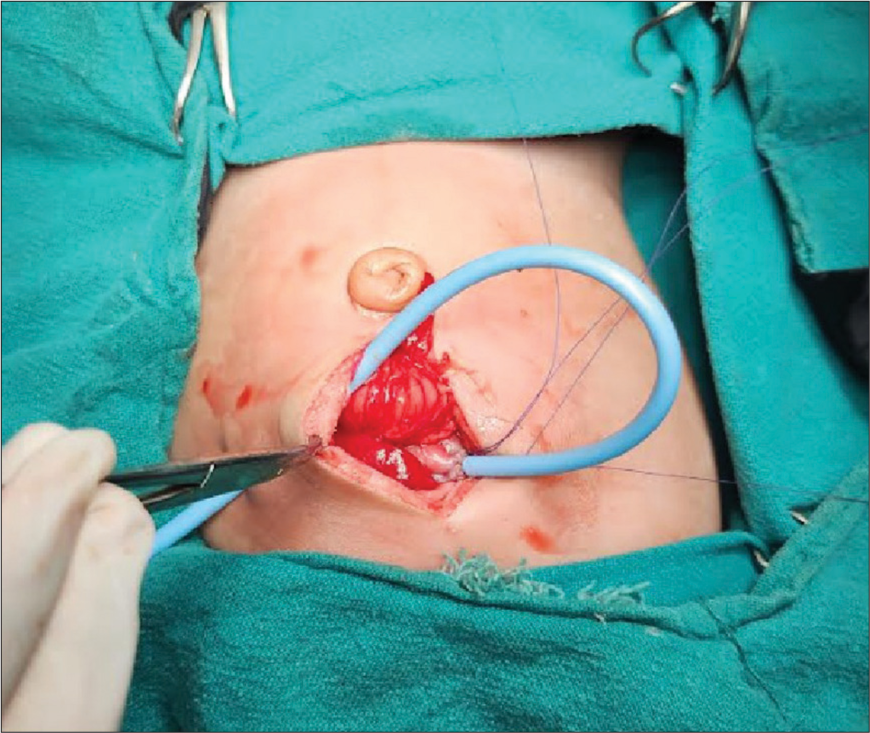
- Intraoperative image of gastrostomy.
Outcome
The post-operative period in the Neonatal Intensive Care Unit was uneventful. There was no event of airway obstruction. The neonate exhibited normal limb movements, with a CRIES (Neonatal Pain Assessment Scale) score of zero indicating effective pain control. The patient was discharged on postoperative day 7. The patient is on continued follow-up at the time of submitting this manuscript and is planned to undergo gastrostomy tube removal once adequate weight gain is achieved.
DISCUSSION
PRS presents significant challenges for anesthesiologists due to craniofacial abnormalities. The airway can be difficult to manage, complicating both ventilation and intubation. Facial deformities make it hard to maintain a facemask seal, and post-operatively, spontaneous airway collapse is a risk. This can arise from pre-existing airway obstruction, obstructive sleep apnea, chronic hypoxia, and heightened opioid sensitivity, particularly in infants with glossoptosis who have central upregulation of opioid receptors.[2] Feeding difficulties, swallowing issues, and gastroesophageal reflux may also lead to bronchial microaspiration and pulmonary infections.[3] Unlike Treacher Collins syndrome, laryngoscopy and intubation in PRS patients are often more difficult in infancy but tend to improve with mandibular growth. In elective settings, fiberoptic intubation is considered the gold standard, though video laryngoscopy and fiberoptic intubation through SGA are also effective alternatives.[1] Our sequential plan to handle this difficult airway scenario in the context of failure to oxygenate is described in [Figure 5].
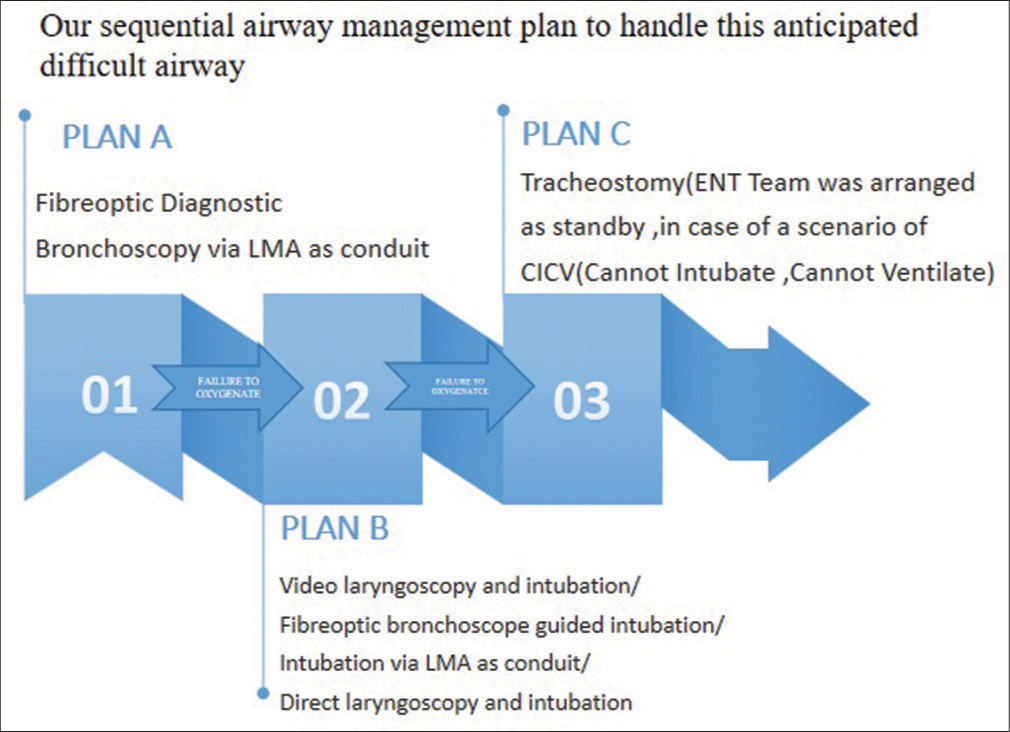
- Our sequential plan to manage the anticipated difficult airway. LMA: Laryngeal mask airway.
Phillips et al. detailed a technique using SGA to address airway obstruction in neonates with PRS. This method allowed for continuous oxygenation and ventilation during nasal bronchoscopic intubation, potentially reducing the risk of oxygen desaturation.[4]
Choosing between regional and general anesthesia in neonates with PRS involves careful consideration due to the potential for airway obstruction during induction, airway manipulation, and the post-operative period. Regional anesthesia, such as spinal anesthesia and peripheral nerve blocks, offers the advantage of maintaining spontaneous breathing, reducing airway obstruction risk, minimizing the need for systemic opioids, avoiding the need for muscle relaxants, and risks associated with their reversal, as well as reducing the incidence of postoperative apnea, a significant concern in neonates, especially those with a history of hypoxic-ischemic injury, as seen in this case. Studies such as general anesthesia and spinal anesthesia (GAS) and Pediatric Anesthesia Neurodevelopment Assessment found no neurodevelopmental differences after a single, short general anesthetic exposure in neonates. However, they did not assess prolonged or repeated exposures, which preclinical studies suggest may cause neuro-apoptosis in a dose and time-dependent manner.[5]
The widespread use of ultrasound has enabled anesthesiologists to explore various fascial planes. In a case series, Breschan et al. found that rectus sheath blocks provided effective intraoperative and post-operative analgesia for most minor upper abdominal surgeries in neonates, who were intubated with muscle relaxants.[6] In contrast, we used intrathecal blockade and post-operative rectus sheath block to maintain spontaneous breathing without muscle relaxants and to ensure post-operative analgesia.
The volume of local anesthetic is described as 1.25 mL/kg for mid-thoracic coverage as per the Green Armitage formula. This volume of local anesthetic in appropriate concentration required for motor blockade for gastrostomy procedure was calculated to be higher compared to the required intrathecal dose of drug as per American Society of Regional Anesthesia recommendations (for children weighing <5 kg, suggested dosage regimen for spinal block with hyperbaric bupivacaine 0.5% is 1 mg/kg).[7,8] Taking into consideration, the reduced risk of local anesthesia systemic toxicity and the advantages of spinal anesthesia such as reduced risk of paralytic ileus and dense analgesia, we successfully conducted gastrostomy under spinal anesthesia.
Surgeons had initially planned only gastrostomy but on the day of the surgery, it was decided to go for bronchoscopy as well. Post-bronchoscopy, we could have gone ahead with general anesthesia with the SGA in situ and employed a caudal block that would have taken care of the intraoperative and post-operative analgesia. However, the requirement of the anesthetic agents and opioids for maintenance and the associated concerns regarding airway collapse as discussed priorly could not have been avoided. We chose to give a Rectus sheath block over caudal postoperatively as it has been demonstrated to have a very effective postoperative analgesic effect. Another option could be a preoperative spinal block followed by postoperative caudal analgesia. However, as we are aware that sacral anomalies are common in these neonates making caudal block difficult, we opted to explore alternative analgesia options, emphasizing the importance of being adaptable and proficient in different approaches while prioritizing patient safety. In Figure 6, we describe the other possible anesthetic management approaches for gastrotomy and the associated adversities of each.
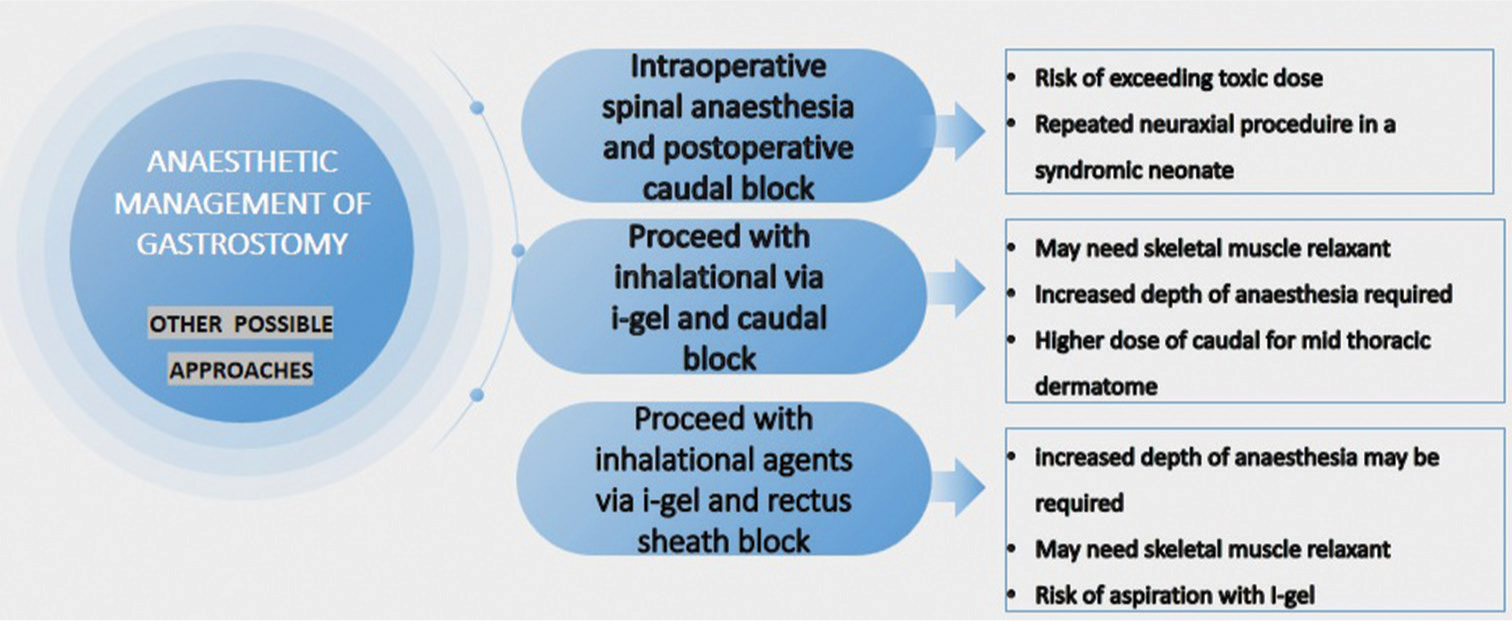
- Comparison of alternative approaches of anesthetic management in this case.
The strengths of this case report include the successful use of combined general and regional anesthesia techniques to manage a neonate with PRS, minimizing the risks associated with airway manipulation and opioid administration, and providing adequate analgesia. The case highlights the importance of tailored anesthetic approaches in neonates with complex airway conditions.
However, employing multiple invasive procedures as in this scenario could be a limitation of our study. Furthermore, the use of total intravenous anesthesia could be employed to avoid pollution while handling the airway. To conduct the surgical procedure under spinal anesthesia, the anesthesiologist should be confident that the procedure will be completed in around 45–60 min, given the short duration of action of intrathecal drugs in neonates. Technical difficulties and failure may be a matter of individual skill and experience. Failure rates of 5–15% have been reported.[9]
We planned our airway approach as per the latest difficult airway guidelines in neonates and infants.[10] Another interesting finding in this case scenario is the abduction deformity of vocal cords which could have contributed to aspiration and subsequent pneumonia. This emphasizes the importance of thorough evaluation of the larynx and vocal cords, particularly before surgical interventions. Furthermore, the finding of posterior vocal adduction defect, in this case, invokes the query of whether it should be labeled a syndromic association or a sequel of a hypoxic perinatal insult. More literature is required to bring light on this matter to plan further management of such patients.
CONCLUSION
This case demonstrates the effectiveness of regional anesthesia techniques in managing neonates with complex airway challenges. The use of spinal anesthesia and peripheral nerve block enabled adequate analgesia and anesthesia, providing an alternative to opioids, minimizing potential complications, and enhancing patient safety. We were also able to successfully maintain the airway during diagnostic bronchoscopy with the SGA as a conduit.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Video available on:
Financial support and sponsorship
Nil.
References
- Managing the Difficult Airway in the Syndromic Child. Contin Educ Anaesth Crit Care Pain. 2015;15:7-13.
- [CrossRef] [Google Scholar]
- Recurrent Hypoxemia in Young Children with Obstructive Sleep Apnea is Associated with Reduced Opioid Requirement for Analgesia. Anesthesiology. 2004;100:806-10. discussion 5A
- [CrossRef] [PubMed] [Google Scholar]
- Swallowing Disorders in Pierre Robin Sequence: Its Correction by Distraction. J Craniofac Surg. 2004;15:934-41.
- [CrossRef] [PubMed] [Google Scholar]
- Placement of a Supraglottic Airway to Overcome Airway Obstruction When Performing Nasal Fiberoptic Intubation in Infants With Pierre Robin Sequence: A Case Series. A A Pract 2020. ;. ;14:e01302.
- [CrossRef] [PubMed] [Google Scholar]
- Fundamentals and Innovations in Regional Anaesthesia for Infants and Children. Anaesthesia. 2021;76(Suppl 1):74-88.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound-guided Rectus Sheath Block for Pyloromyotomy in Infants: A Retrospective Analysis of a Case Series. Paediatr Anaesth. 2013;23:1199-204.
- [CrossRef] [PubMed] [Google Scholar]
- Caudal Epidural Blocks in Paediatric Patients: A Review and Practical Considerations. Br J Anaesth. 2019;122:509-17.
- [CrossRef] [PubMed] [Google Scholar]
- Paediatric Regional Anaesthesia: Updates in Central Neuraxial Techniques and Thoracic and Abdominal Blocks. BJA Educ. 2019;19:126-34.
- [CrossRef] [PubMed] [Google Scholar]
- Spinal Anesthesia in Children: A Review. J Anaesthesiol Clin Pharmacol. 2014;30:10-8.
- [CrossRef] [PubMed] [Google Scholar]
- Airway Management in Neonates and Infants: European Society of Anaesthesiology and Intensive Care and British Journal of Anaesthesia Joint Guidelines. Br J Anaesth. 2024;132:124-44.
- [CrossRef] [PubMed] [Google Scholar]







