Translate this page into:
Perioperative Cardiac Dysrhythmias In The Neonate – Bradycardia and Tachycardia
*Corresponding author: Dr. Shreshtha Jha, Department of Anesthesia, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, India. shreshthajha2410@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Jha S. Perioperative Cardiac Dysrhythmias In The Neonate – Bradycardia and Tachycardia. J Neonatal Crit Care Anesth. 2024;1:32-8. doi: 10.25259/JNCCA_9_2024
Abstract
Managing neonates in a perioperative setting is a challenging task. Neonates differ from pediatric and adult patients in many ways. Anesthesiologists should have knowledge and skills in the recognition and prompt treatment of cardiac dysrhythmias in these babies. We emphasize the etiology, recognition, implications, and management of the most common types of arrhythmias, bradycardia, and tachycardia in neonates in the perioperative period.
Keywords
Neonate
Perioperative
Bradycardia
Tachycardia
INTRODUCTION
Due to advances in neonatal care and the availability of neonatal intensive care units (NICU), there is improved survival of neonates. Preterm babies, American Society of Anesthesiologists (ASA) class III/IV, those posted for emergency surgery, and those with congenital heart diseases are extremely prone to dysrhythmias in the perioperative period that can adversely impact their survival after surgery. Hence, it is important that anesthesiologists have knowledge and skills in the recognition and management of dysrhythmias in the perioperative period as a part of the Perioperative Neonatal Resuscitation (PoNR) Protocol.
NEONATAL BRADYCARDIA
Innervation of the developing heart and the neurotransmitters
The heart has both sympathetic and parasympathetic innervation. The sensory (afferent) nerves take information from the heart to the brain through both sympathetic and parasympathetic pathways. The motor (efferent) brings signals from the brain to the heart through both sympathetic and parasympathetic pathways. The parasympathetic supply is by the vagus nerve, with acetylcholine being the primary neurotransmitter. Sympathetic supply is by the T1-T4 nerves, with nor-epinephrine as the primary neurotransmitter. Although the primitive unmyelinated vagus nerve develops first, it does not have much influence before the 25th week of gestation. Maturation occurs in the 3rd trimester leading to a steep increase in vagal tone near term. Hence, while sympathetic innervation is incomplete and poorly developed, parasympathetic innervation is preponderant in neonates. This explains the vagal predominance in neonates, leading to a higher frequency of bradycardia in response to stressors such as hypoxia and laryngoscopy; unlike children and adults. They also have poor sensitivity to sympathomimetic agents, both intrinsic and extrinsic. This parasympathetic predominance diminishes by 6 months of age. Heart rate in newborns and neonates is in the range of 120–160 beats/min. There have been varying definitions of bradycardia in a neonate with a heart rate <80 or 60 considered bradycardia requiring intervention. However, considering the perioperative scenario, a heart rate <100 beats/min can be considered neonatal bradycardia.[1-4] The conduction intervals as seen on an electrocardiogram (ECG) are also shorter than in children and adults. PR interval ranges between 70 and 140 milliseconds (ms) (0.07–0.14 s) with a mean of 100 ms (0.10 s).[5]
Classification of bradycardia
Primary bradycardia is due to intrinsic cardiac causes leading to cardiac electrical system dysfunction. For example, Congenital or acquired heart conditions that slow the spontaneous rate of depolarization of the heart’s pacemaker cells or slow the conduction through the conduction system of the heart such as sick sinus syndrome and atrioventricular (AV) block
Secondary bradycardia is due to extrinsic factors affecting normal heart function such as hypoxia, hypothermia, vagal stimulation, certain medications, and electrolyte imbalances which slow the sinus node pacemaker and/or slow conduction through the AV node.
Bradycardia which is associated with a rhythm disturbance is called bradyarrhythmia.[6]
Implication of neonatal bradycardia
Cardiac output is a product of stroke volume and heart rate and in neonates, as the developing heart is less compliant and poorly contractile, the stroke volume cannot increase much. Hence, heart rate is the predominant factor determining the cardiac output. Bradycardia will lead to a fall in cardiac output, hypotension, and poor peripheral circulation that may further lead to serious arrhythmias and even cardiac arrest. Therefore, prevention, prompt recognition, and management of perioperative neonatal bradycardia are of utmost importance.[7]
Risk factors for perioperative bradycardia in neonates
ASA physical status – ASA 3 or more
Emergency surgery
Congenital heart disease
Ex-preterm neonates.
Causes of neonatal bradycardia in the perioperative period can be broadly classified as intrinsic (cardiac causes) or extrinsic [Table 1]. As an anesthesiologist, one must know the extrinsic factors that can contribute to bradycardia in neonates in the perioperative period. The most commonly encountered causes are airway-related and drugs.[3,7-9]
| Airway related |
| During laryngoscopy: Due to activation of the laryngeal reflex stimulating the already heightened vagal tone. |
| Hypoxemia following inadequate ventilation and oxygenation: |
| Laryngospasm and bronchospasm |
| Soft-tissue airway obstruction |
| Esophageal or endobronchial intubation |
| Endotracheal tube kinking, misplacement, or accidental extubation |
| Medications related |
| Halothane-induced cardiovascular depression |
| Propofol |
| Opioids |
| Non-depolarizing muscle relaxants |
| Succinylcholine |
| Neostigmine |
| Intravascular injection of local anesthetic |
| Electrolyte imbalance – Hyperkalemia, hypocalcemia, and hypomagnesemia |
| Significant blood loss |
| Hypothermia |
| Surgery – procedures causing vagal stimulation – bowel traction, pressure over the eyeball, neck region, chest, abdominal insufflation as for laparoscopic surgery |
Rhythm identification
Identifying the abnormal rhythm is the first step toward proper management. Common abnormal bradycardia rhythms encountered in the perioperative period are described below [Table 2 and Figure 1].[6,10]
| Sinus Bradycardia – Normal sinus rhythm with a slow heart rate. |
| Sinus block (Sinus node arrest) – Absence of normal P wave on ECG. Due to the absence of sinus node activity, the heart generates escape beats that originate from the atria, AV node, or ventricles. |
| First-degree atrioventricular block – PR interval is longer than 0.10 s.[5] |
| Second-degree atrioventricular block – there is blockade of some but not all atrial impulses before they reach the ventricles |
| Mobitz type I block/Wenckebach phenomenon – This occurs at the level of the AV node. Progressive prolongation of PR interval until an atrial impulse is not conducted to the ventricles and a QRS complex is dropped, that is, the P wave is not followed by the QRS complex. This cycle typically repeats. |
| Mobitz type II – This occurs below the level of the AV node. There is non-conduction of some atrial impulses to the ventricles but without any change in the PR interval of conducted impulses. The PR interval is the same with an intermittently dropped QRS complex. |
| Third-degree atrioventricular block (complete heart block) – None of the atrial impulses are conducted to the ventricles. P wave and QRS complex are not coordinated with each other. This is also known as atrioventricular dissociation. |
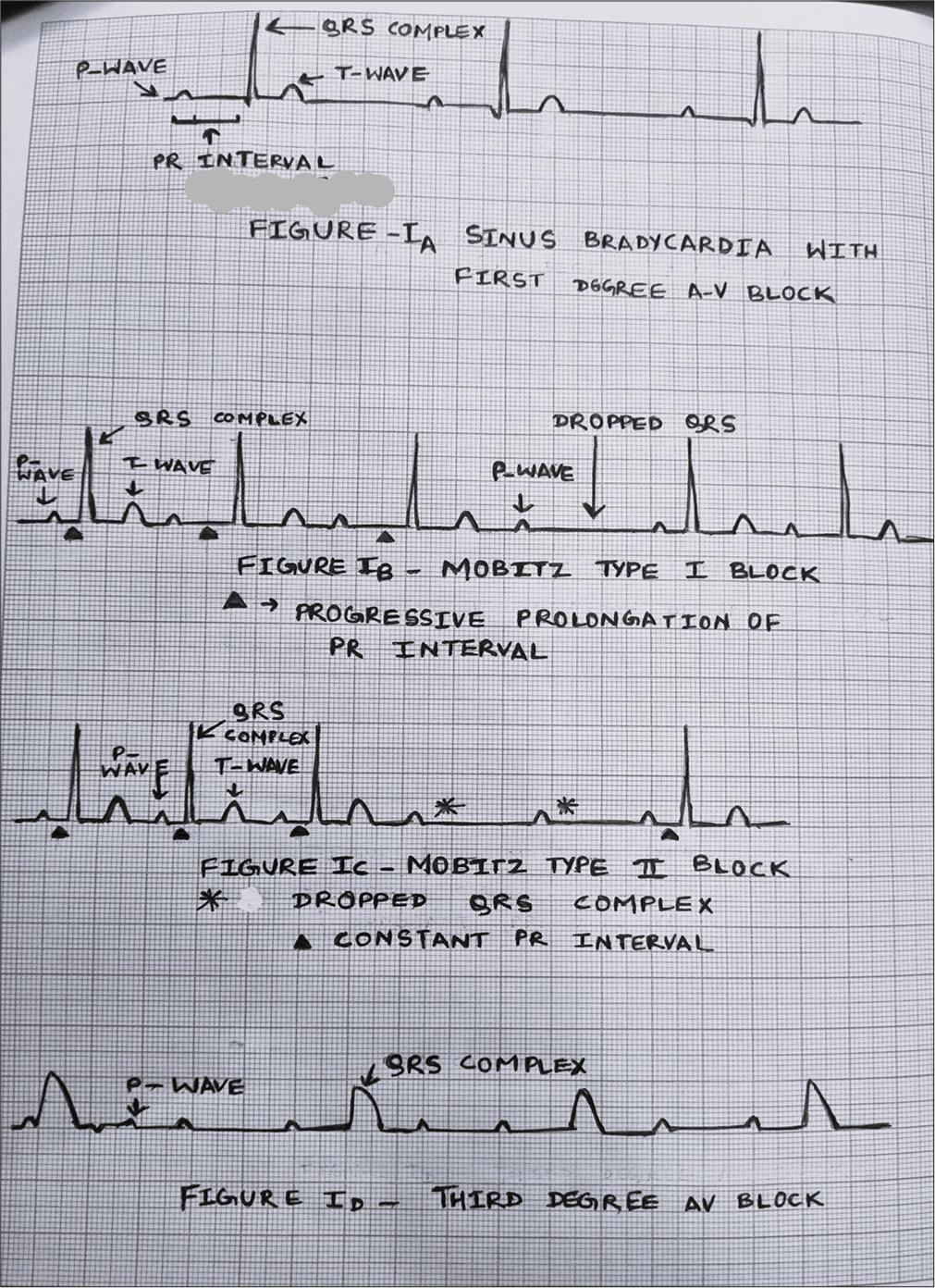
- Bradyarrhythmia.
Management principles
The management, in the perioperative period, depends upon whether the bradycardia is symptomatic or asymptomatic, and whether the heart rate has fallen to less than 100 beats/min [Flowchart 1].
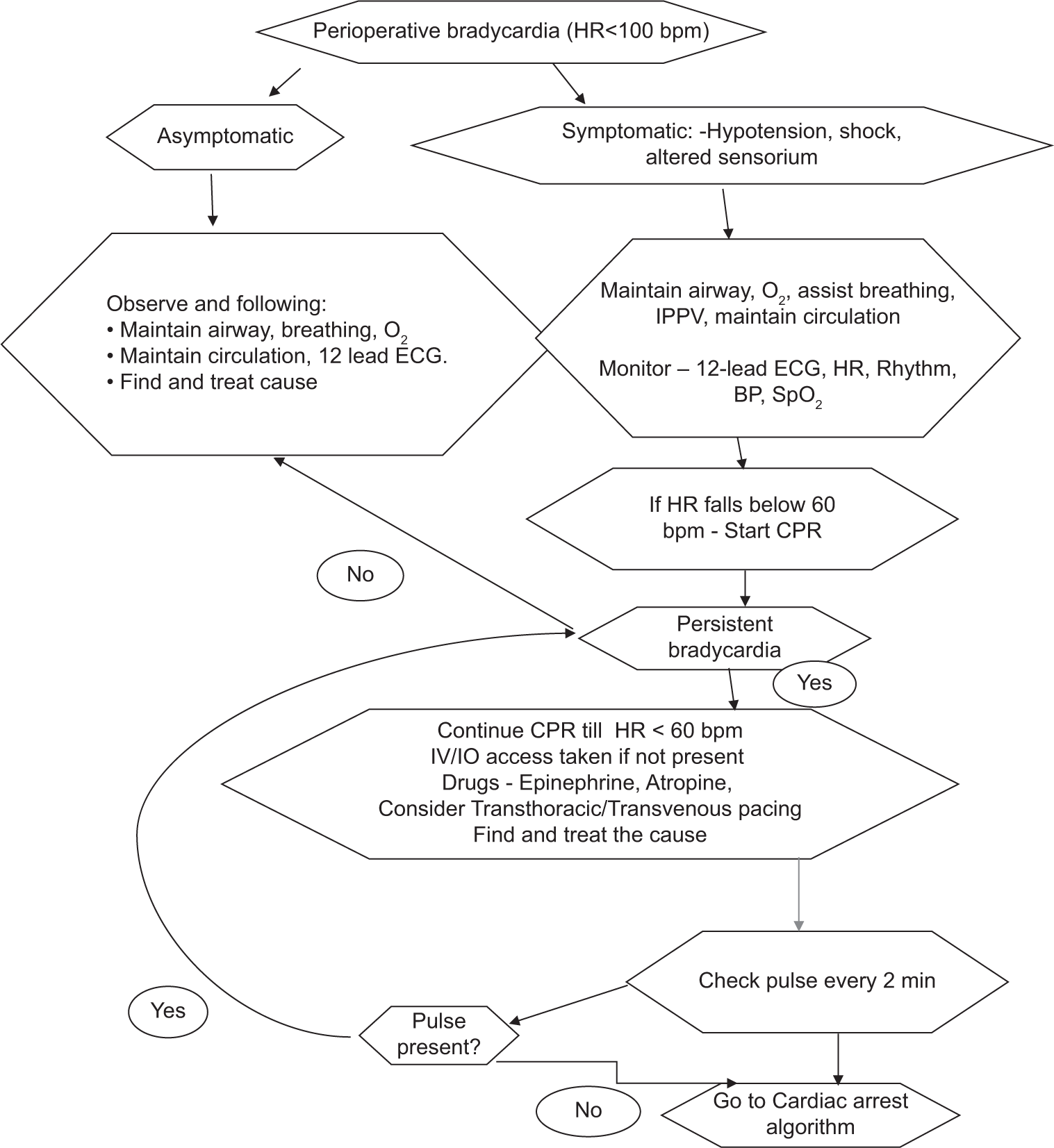
- Management of perioperative neonatal bradycardia. O2: Oxygen, ECG: Electrocardiogram, IPPV: Intermittent positive pressure ventilation, HR: Heart rate, SpO2: Oxygen saturation, BP: Blood pressure, NIBP: Noninvasive blood pressure, CPR: Cardiopulmonary resuscitation, IV/IO: Intravenous/intra-osseous, RR: Interval between two R waves on ECG, H/o: History of, bpm: Beats per minute.
In the perioperative setup, exposure to deleterious medications, drug errors, surgical stimulus, and vagal stimulus should be sought for, identified, and discontinued. Potentially reversible and treatable causes should be recalled with the help of mnemonic for “6 Hs and 4 Ts” and promptly identified and treated. The 6 H’s include hypovolemia, hypoxia, hydrogen ion (acidosis), hypoglycemia, hypo-/hyperkalemia, and hypothermia. The 4 T’s include tension pneumothorax, tamponade (cardiac), toxins, thrombosis (coronary), and thrombosis (pulmonary).[6,9,10]
Drugs used in the management of bradycardia
1. Epinephrine IV/IO dose: 0.01 mg/kg. It can be repeated every 3–5 min. If IV/IO access is not available, it can be administered through the endotracheal route, at a dose of 0.1 mg/kg
Indication: Any symptomatic bradycardia that persists despite effective oxygenation, ventilation, and cardiopulmonary resuscitation
Mechanism of action: It acts through α-adrenergic and β-adrenergic mechanisms.
The effectiveness of epinephrine may be reduced due to acidosis and hypoxia. Therefore, supporting airway, ventilation, oxygenation, and perfusion are important.
For persistent bradycardia, a continuous infusion of epinephrine in a dose of 0.1–0.3 mcg/kg/min can be considered, especially if the baby has responded to a bolus of epinephrine.
2. Atropine IV/IO dose: 0.02 mg/kg. It may be repeated once. The minimum single dose is 0.1 mg. The maximum single dose is 0.5 mg.
Indication: Increased vagal tone, first-degree AV block, and Mobitz type I block. If there is poor or no response to drugs, one must seek an expert cardiology opinion. Transthoracic/transvenous pacing can be considered, especially if bradycardia is due to abnormal SA node function or Mobitz type 2/complete heart block. This is done under sedation.[6,9,10]
TACHYARRHYTHMIA
Tachyarrhythmias are rapid abnormal rhythms originating in either the atria or ventricles or sometimes junctional.[6]
Implication – With increased heart rate, the diastolic phase is shortened, due to which ventricles are unable to fill completely, leading to reduced end-diastolic left ventricular volume, low stroke volume, and low cardiac output. The coronary arteries also receive less blood, which leads to reduced cardiac perfusion. Rapid heart rate also increases myocardial oxygen demand.[6,10]
Types of tachycardia
This includes narrow complex and wide complex tachycardia.[6,10-12]
-
Narrow complex tachycardia – QRS duration ≤0.09 s. They originate at or above the AV node and are also known as supraventricular tachycardia. This includes sinus tachycardia, paroxysmal supraventricular tachycardia (PSVT), atrial flutter, and atrial fibrillation (AF). Atrial flutter and fibrillation are less common in neonates. There are three mechanisms behind narrow complex tachycardia:
Re-entry tachycardia – This occurs due to the presence of a re-entry circuit which can be at the level of the AV node, functional re-entry circuit (atrioventricular node re-entry tachycardia, that is, AVNRT), or through an accessory anatomical pathway, the bundle of Kent (AV re-entry tachycardia [AVRT]). These two comprise PSVT. Re-entry can also be within the atrium producing atrial flutter or fibrillation.
Automatic tachycardia – Results from an enhanced automatic focus present within the sino-atrial (SA) node as in sinus tachycardia or foci elsewhere in the atria producing ectopic atrial tachycardia and multifocal atrial tachycardia.
Triggered tachycardia – Due to a triggered activity. It is a much less common mechanism except in the setting of drug toxicity, especially digoxin overdose.
Often it is required to distinguish between sinus tachycardia and PSVT, commonly encountered tachyarrhythmias.
Sinus tachycardia will usually have a fast heart rate (generally <220/min), has a slow onset, and is often associated with reversible causes such as fever, hypovolemia, and stress. Heart rate may vary with activity, and a P wave is usually present on ECG.[6,10]
In PSVT, a fixed heart rate of >220/min usually, in a neonate, has an abrupt onset and equally abrupt offset. The P wave is absent on ECG or may be retrograde or inverted. In typical AVNRT, retrograde P waves occur early (buried in QRS) and are not seen, or partially seen (pseudo-R’ wave at the terminal portion of QRS complex). In AVRT, retrograde P waves occur later (prolonged RP interval).
Wide complex tachycardia – QRS duration >0.09 s. The same basic mechanisms of automaticity, re-entry, and triggered tachycardia cause wide complex tachycardia. This includes ventricular tachycardia and supraventricular tachycardia with aberrant conduction. These are uncommon in neonates but are more serious.
Management principles
Management depends upon whether the neonate is asymptomatic or symptomatic and on the type of tachycardia (narrow complex or wide complex) [Flowchart 2 and Figure 2].[6,9,10]
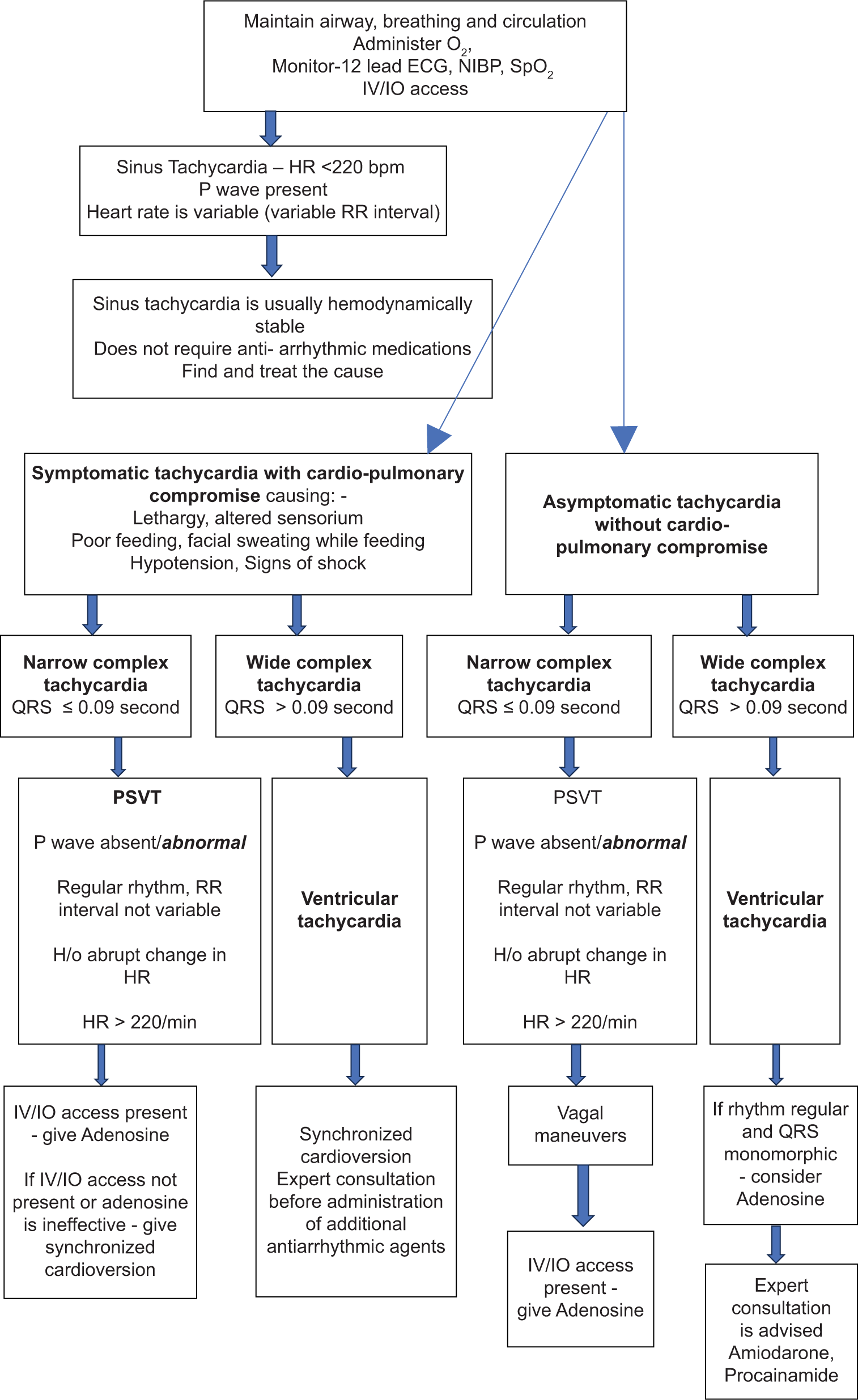
- Neonatal tachycardia with a pulse. O2: Oxygen, ECG: Electrocardiogram, HR: Heart rate, SpO2: Oxygen saturation, BP: Blood pressure, NIBP: Noninvasive blood pressure, IV/IO: Intravenous/intra-osseous, RR: Interval between two R waves on ECG, bpm: Beats per minute, H/o: History of, PSVT: Paroxysmal supraventricular tachycardia.
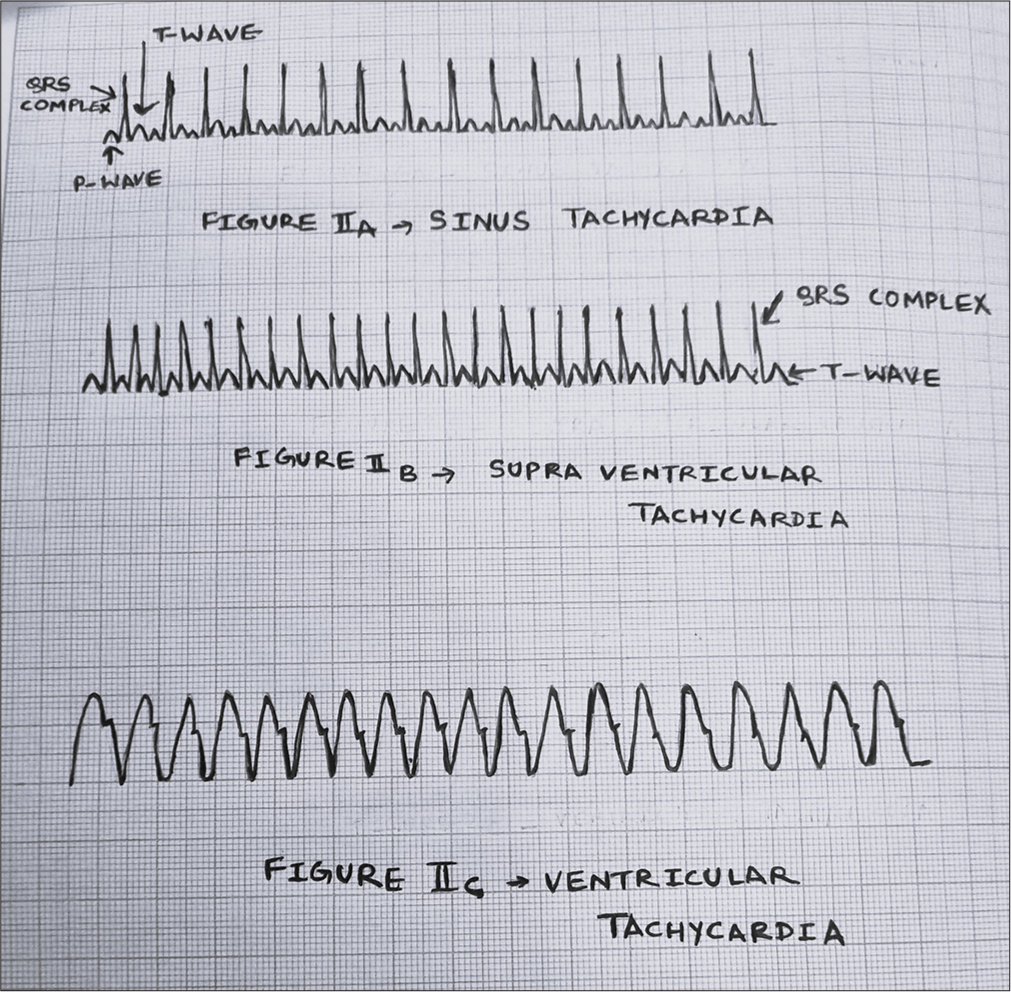
- Tachyarrhythmia.
Vagal maneuvers
Vagal maneuvers are done to stimulate the parasympathetic nervous system and reduce the heart rate, such as by application of ice to the face. Fill a small plastic bag with a mixture of ice and water and apply it to the upper half of the baby’s face for 15–20 s. Be careful to not occlude the nose or mouth. Carotid sinus massage may be difficult to perform in the neonatal age group. Do not use ocular pressure as it may produce retinal injury.[6]
Drugs and dosages
-
Adenosine IV/IO – 1st dose 0.1 mg/kg (maximum 6 mg) is given as a rapid push. 2nd bolus of 0.2 mg/kg (maximum 12 mg) as a rapid bolus can be repeated after 1–2 min of first dose. Each bolus must be flushed with 0.5–1 mL/kg normal saline.
Mechanism of action – It blocks conduction through the AV node temporarily, for about 10 s
Indication – It is effective for the treatment of PSVT (caused by re-entry) mechanism.
It is not effective for atrial flutter, AF, and tachycardia caused by mechanisms other than re-entry through the AV node.
-
Amiodarone IV/IO – dose 5 mg/kg to be given over 20–60 min
Mechanism of action – It inhibits α and β adrenergic receptors which cause vasodilatation and AV node suppression. It also inhibits outward potassium current and prolongs QT interval. It inhibits sodium channels which slows conduction in the ventricles and prolongs QRS duration
Procainamide IV/IO – Dose 15 mg/kg over 30–60 min
Note –
Amiodarone should not be administered rapidly as it may cause hypotension due to vasodilatation and polymorphic ventricular tachycardia (VT) by prolonging the QT interval
Amiodarone and procainamide should not be administered together as both cause QT prolongation
Drugs such as short-acting β blockers and digoxin have been used for the treatment of supraventricular tachycardia in babies but they should be reserved for use after expert consultation
Verapamil should not be used in neonates as it may cause refractory hypotension and cardiac arrest
If the wide complex tachycardia has a uniform QRS morphology and regular RR interval in an otherwise hemodynamically stable neonate, adenosine can be considered. This is because if it is VT, then adenosine will not be effective but will also do no harm. However, if it is due to supraventricular tachycardia (SVT) with aberrancy, adenosine will be effective.[6]
SYNCHRONIZED CARDIOVERSION
If the baby does not respond to the above drugs, proceed with cardioversion
Sedation should be given before synchronized cardioversion but does not delay cardioversion for it.
Start with an energy of 0.5–1 J/kg. If it is not effective, increase to 2 J/kg.[6]
AFTER CARE
As these babies are prone to recurrence of arrhythmias, it is very important to find the cause and treat it. These babies should be kept in the neonatal intensive care unit for monitoring and further management. Cardiology reference should be sought and the baby should be kept under observation and in follow-up.
CONCLUSION
As neonates are different from pediatric and adult patients, it is important to have knowledge and skills in the management of cardiac dysrhythmias in the perioperative period as a part of PoNR protocol. An algorithm-based approach can be used for this. Furthermore, it is equally important to follow up with these babies and to take care of reversible factors, so as to prevent the recurrence of arrhythmias. In case, the cause can be diagnosed, appropriate specific treatment should be given.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Development of the Cardiovascular System In: Andropoulos DB, Gregory GA, eds. Gregory's Pediatric Anesthesia (6th ed). Hoboken, NJ: Wiley-Blackwell; 2020. p. :76-99.
- [CrossRef] [Google Scholar]
- Prevalence of Bradycardia After Induction of General Anesthesia and Associated Factors Among Surgical Pediatric Patients. A Prospective Observational Study. Pediatric Health Med Ther. 2023;14:419-34.
- [CrossRef] [PubMed] [Google Scholar]
- Autonomic Nervous System in the Neonate In: Saha U, ed. Clinical Anesthesia for the Newborn and the Neonate (1st ed). Singapore: Springer; 2023. p. :458-76.
- [CrossRef] [Google Scholar]
- Guidelines for the Interpretation of the Neonatal Electrocardiogram. A Task Force of the European Society of Cardiology. Eur Heart J. 2002;23:1329-44.
- [Google Scholar]
- Pediatric Advanced Life Support Provider Manual United States: American Heart Association; 2016. p. :239-75.
- [Google Scholar]
- Bradycardia in Children During General Anaesthesia In: Breijo-Marquez FR, ed. Cardiac Arrhythmias - New Considerations. London: InTech; 2012. p. :343-56.
- [Google Scholar]
- Clinical Complications in Pediatric Anesthesia In: Andropoulos DB, Gregory GA, eds. Gregory's Pediatric Anesthesia (6th ed). Hoboken, NJ: Wiley-Blackwell; 2020. p. :1118-49.
- [CrossRef] [Google Scholar]
- Cardiopulmonary Resuscitation In: Davis PJ, Cladis FP, eds. Smith's Anesthesia for Infants and Children (9th ed). St. Louis, Missouri: Elsevier; 2017. p. :1236-81.
- [CrossRef] [Google Scholar]
- PALS Pediatric Advanced Life Support- Provider Handbook Las Vegas, NV: Satori Continuum Publishing; 2021. p. :35-43.
- [Google Scholar]
- Part 4: Pediatric Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142:S469-523.
- [CrossRef] [PubMed] [Google Scholar]
- Emergency diagnosis and management of pediatric arrhythmias. J Emerg Trauma Shock. 2010;3:251-60.
- [CrossRef] [PubMed] [Google Scholar]






