Translate this page into:
Ligation of Patent Ductus Arteriosus in Preterm Infant with Comorbidities: An Unforgotten Nightmare
*Corresponding author: Minati Choudhury, Professor, Department of Cardiac Anaesthesia and Critical Care, All India Institute of Medical Sciences, New Delhi, India. minati.2002@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Choudhury M, Gupta A, Krithika KG, Bharadwaj V, Devagourou V. Ligation of Patent Ductus Arteriosus in Preterm Infant with Comorbidities: An Unforgotten Nightmare. J Neonatal Crit Care Anesth. 2024;1:46-8. doi: 10.25259/JNCCA_5_2023
Abstract
Patent ductus arteriosus (PDA) is a common congenital anomaly in preterm infants. We report here a case of 28-day-old preterm infant, weighing 1.6 kg, with significant PDA, having bronchopulmonary dysplasia, necrotizing enterocolitis, intraventricular hemorrhage, hydrocephalus, and meningitis; presented for PDA ligation in view of prolonged ventilator dependence, non-responsiveness to pharmacological therapy for PDA closure, and continuous worsening of hemodynamics due to the presence of large PDA.
Keywords
Neonate
anesthesia
Patent ductus arteriosus
Premature infant
Bronchopulmonary dysplasia
Necrotizing enterocolitis
Interventricular hemorrhage
INTRODUCTION
Patent ductus arteriosus (PDA) is a common congenital anomaly with 15–37% incidence in preterm newborns weighing <1750 g.[1] The variety of comorbidities such as bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH), and prolonged ventilator dependence can pose a major challenge in the perioperative management of PDA ligation. Here, we report a case of 28-day-old infant, weighing 1.6 kg, who presented for PDA ligation in view of prolonged ventilator dependence and significant PDA on echocardiography causing unstable hemodynamics. There is a paucity in the literature about the discussion of such kind of case from an anesthesiologist’s point of view.
CASE REPORT
A 750 g female baby, the 2nd twin, born preterm with a gestational age of 27 weeks, was admitted to the neonatal intensive care unit (NICU) and underwent mechanical ventilation as she was suffering from respiratory distress syndrome (RDS). An early rescue surfactant was given in view of RDS but the baby developed bronchopulmonary dysplasia (BPD), pulmonary hemorrhage, and ventilator dependence in due course of time. The baby had sudden worsening hypotension on day 12th. She was managed with inotropes (dopamine, dobutamine at a dose of 5 μ/kg body weight each) and injection hydrocortisone at a dose of 2.5 mg/kg and was shifted to pediatric cardiology intensive care unit for further management of worsening hemodynamics. Ultrasonography of the cranium revealed left-sided grade II IVH. Later, she developed post-IVH hydrocephalus with meningitis, and multiple spinal taps were performed. The baby had gradual abdominal distension and bluish discoloration of the abdomen on 3rd week of the postnatal period. Abdominal X-ray [Figure 1] showed distended bowel loops and gas under the diaphragm, suggestive of NEC/abdominal perforation for which a gloved drain was secured, antibiotics as well as bowel rest were initiated. 2-D transthoracic echocardiography revealed large PDA (3mm) with low-velocity flow, severe pulmonary artery hypertension and left ventricular volume overload. Medical treatment for PDA closure with intravenous (IV) paracetamol 15 mg/kg every 6 hourly for 72 h was tried but failed. No pharmacological intervention was done for pulmonary hypertension. On the 28th day of life, due to non-improvement of heart failure, she was posted for PDA ligation. She weighed 1.6 kg at this point in time. The immediate preoperative arterial blood gas findings are as follows: Partial pressure of oxygen 65 mmHg, partial pressure of carbon dioxide (PaCO2) 38 mmHg, bicarbonate 20 meq/L, sodium 133 meq/L, K 3.8 meq/L, and calcium 2.5 mmoL/L. She was ventilated with a tidal volume of 6 mL/kg, respiratory rate of 40/min, positive end-expiratory pressure (PEEP) of 6 cm water (H2O), and I: E ratio of 1:1.5. She was kept nil per oral and hydrated with 4 mL/kg/h IV fluids. OT was kept warm. IV rocuronium bromide (1.5 mg) and fentanyl (5 μg) were given after connecting the endotracheal tube (3.0 mm ID) to the anesthesia machine. An additional 26 G IV cannula in the right arm for infusion and a left femoral arterial (24G cannula) line for invasive monitoring were secured. Her baseline heart rate was 165/min, blood pressure 50/36 mmHg, and arterial oxygen saturation (SaO2) of 85% on both the upper (right, preductal) and lower extremity (post-ductal). The end-tidal carbon dioxide was 42 mm Hg and peak airway pressure was 21 mmHg. She was ventilated with a tidal volume of 6mL/kg, respiratory rate 40/min, PEEP 6 cm H2O, and I: E ratio 1:1.5. The target SaO2 was aimed at 90%. Continuous monitoring of cerebral oxygenation was done by near-infrared spectrometry (NIRS) (INVOS™ 5100C, Somanetic, Troy, MI). She was positioned for left thoracotomy. During retraction of the left lung, she had multiple episodes of desaturation and bradycardia, which were relieved with intermittent positive pressure bag hyperventilation, and release of a surgical retractor. PDA was exposed slowly and ligated cautiously. She developed severe bradycardia (HR 30/min), hypotension (systolic blood pressure 30 mmHg), and desaturation (SpO2 <40%) after PDA ligation during rib approximation for closure. The procedure was stopped and one of the sutures from the mediastinal pleura was removed. IV atropine was given at a dose of 20 μ/kg twice. The episode soon terminated and vitals remained stable thereafter. The NIRS values remained between 58 and 68% throughout except for a decline to 40% for 2 min during the period of bradycardia and hypotension. The baby was shifted to the NICU of the cardiac surgical intensive care unit for elective ventilation on inotropic support (dobutamine 5 μ/kg/min). The NICU stay lasted for one month due to presence of ventricular dysfunction and respiratory issues. Due to non-improvement of oxygen saturation, sildenafil (1 mg/kg/day) was started on postoperative day 4. There was a gradual improvement in SaO2 by day 8 and ventricular function by day 15. She was extubated on postoperative day 20, off oxygen on the 29th day. She was discharged on the 68th day of life, with 95% SaO2 on air.
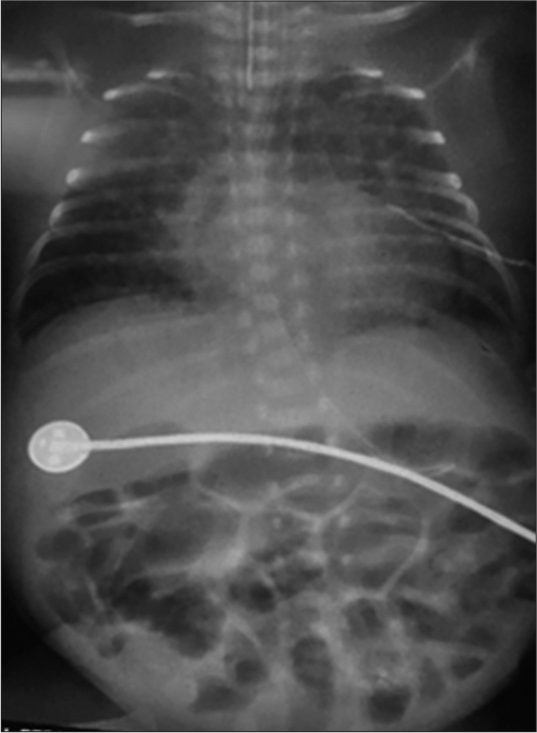
- X-ray chest-abdomen of the neonate showing hazy opaque lungs, diffuse interstitial thickening as result of bronchopulmonary dysplasia, cardiomegaly, and pulmonary plethora due to patent ductus arteriosus, generalized distension of intestinal loops as a feature of necrotizing enterocolitis.
DISCUSSION
The spontaneous closure of PDA is less frequent and delayed in preterm infants, especially with low birth weight. The decision on PDA treatment in premature newborns is a matter of debate as it is considered a physiological alteration rather than a pathological condition. However, association of PDA with increased chances of BPD, NEC, and IVH has been considered as the need for its early closure by neonatologists for decades.[2,3] In our patient, failure of medical therapy, echocardiographic evidence of a large defect, and ongoing ventilatory requirements were reasons for surgical ligation of PDA. BPD is a chronic lung disease as a consequence of severe RDS, which needs prolonged ventilator occupancy and oxygen dependency.[4,5] Necrotizing enterocolitis is an inflammatory condition of the intestine, extending systemically to affect distant organs such as the brain causing neurodevelopmental delay. Perforated NEC in low-birth-weight newborns is one of the most common surgical conditions encountered in NICU.[6-8] Interventricular hemorrhage can lead to hydrocephalus and meningitis, also causing neurodevelopmental delay if it is progressive.[9] We did continuous NIRS monitoring during the intraoperative period and intermittently during postoperative hospital stay as it is postulated that values below 40–45% have deleterious effects on cerebral blood flow and growth.[10] It is a well-known fact that both the extremes of blood pressure have important implications in the development of IVS. In this scenario, the use of NIRS may help to identify the vulnerable neonates and facilitate judicious use of inotropes to minimize fluctuations in cerebral perfusion. It is widely known that acute fluctuations of PaCO2 are associated with alterations in cerebral blood flow; therefore, the use of NIRS in mechanically ventilated extremely preterm infants may help to detect and correct the changes before injury can occur.[10] It may help to detect or potentially ameliorate cerebral insults related to IVH.[11]
Our patient had multiple episodes of bradycardia and desaturation during surgical exposure for PDA ligation, due to a combination of factors leading to hypoxia. Bradycardia and hypotension during rib approximation may have been due to vagal irritation secondary to irritation by mediastinal pleural suture. Zarrabi et al, reported a case of severe bradycardia and hypotension in a child in which the vagal trunk was entrapped within the suture line.[12] First, BPD and pulmonary immaturity can lead to cardiorespiratory failure. Second, abdominal distension due to NEC decreases lung compliance. The left lung compression during surgical exposure may cause intrapulmonary hemorrhage and/or atelectasis, which the anesthesiologist should be aware of during perioperative management. Dependent position and surgical pads under the already diseased lung further compromise respiratory mechanics.
CONCLUSION
Preterm neonates with ventilator dependence requiring surgical PDA ligation are not common as most of the cases respond to medical therapy. Secondly, managing a low birth weight preterm neonate is more challenging compared to a term normal weight neonate, because the former is at a higher risk of complications due to immature organ system, vulnerability to hypothermia, hypoglycemias and infections. Finally, a preterm neonate with so many comorbidities poses a different challenge altogether for the anesthesiologist. These premature infants are at increased risk of hypoxia leading to hemodynamic instability during surgical exposure for PDA ligation. Anesthesiologists managing these babies should be extra-cautious about cardiorespiratory compromise during PDA ligation and have pre-knowledge of the associated morbidities affecting various organ systems. Monitoring is equally important to manage these patients till discharge due to several issues related to prematurity.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Patent Ductus Arteriosus in Preterm Infants. Indian Pediatr. 2011;17:301-8.
- [CrossRef] [Google Scholar]
- Patency of the Ductus Arteriosus in the Premature Infant: Is It Pathologic? Should It Be Treated? Curr Opin Pediatr. 2004;16:146-51.
- [CrossRef] [Google Scholar]
- Patent Ductus Arteriosus Ligation in Premature Infants: Who Really Benefits, and at What Cost? J Pediatr Surg. 2007;42:69-75.
- [CrossRef] [Google Scholar]
- Bronchopulmonary Dysplasia in Preterm Infants Born at Less than 32 Weeks of Gestation. Glob Pediatr Health. 2016;3:2333794X16668773.
- [CrossRef] [Google Scholar]
- Bronchopulmonary Dysplasia: An Enduring Challenge. Pediatr Rev. 2002;23:349-58.
- [CrossRef] [Google Scholar]
- Necrotizing Enterocolitis: The Role of Hypoxia, Gut Microbiome and Microbial Metabolites. Int J Mol Sci. 2023;24:2471.
- [CrossRef] [Google Scholar]
- Outcome of Perforated Necrotizing Enterocolitis in the Very Low-Birth Weight Neonate May be Independent of the Type of Surgical Treatment. Am Surg. 2001;67:752-6.
- [CrossRef] [Google Scholar]
- White Matter Injury in Infants with Intraventricular Haemorrhage: Mechanics and Therapies. Nat Rev Nerol. 2021;17:199-214.
- [CrossRef] [Google Scholar]
- Monitoring Cerebral Oxygenation of the Immature Brain: A Neuroprotective Strategy? Pediatr Res. 2018;84:159-64.
- [CrossRef] [Google Scholar]
- Monitoring and Management of Brain Hemodynamics and Oxygenation. Handb Clin Neurol. 2019;162:295-314.
- [CrossRef] [Google Scholar]
- Profound Bradycardia Following Patent Ductus Arteriosus Closure; A Rare but Correctable Event. Bull Emerg Trauma. 2013;1:130-2.
- [Google Scholar]






