Translate this page into:
Anesthetic Considerations in Neonates with Tracheoesophageal Fistula: Navigating Challenges for Successful Outcomes
*Corresponding author: Purnima Bhanot, Department of Anaesthesia, Dayanand Medical College and Hospital, Ludhiana, Punjab, India. 1996purn@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bhanot P, Singh U, Grewal AG, Grewal G, Grewal A. Anesthetic Considerations in Neonates with Tracheoesophageal Fistula: Navigating Challenges for Successful Outcomes. J Neonatal Crit Care Anesth. 2024;1:42-5. doi: 10.25259/JNCCA_6_2024
Abstract
Tracheoesophageal fistula (TEF) is an anomalous connection between the trachea and esophagus with an incidence of 1 in every 2500 live births making it one of the most common congenital anomalies seen in major neonatal surgical centers. If left undiagnosed and untreated, it may lead to neonatal morbidity and early mortality. This case report focuses on the challenges faced in the anesthetic management of neonates with TEF, their airway management, and ventilation strategies in a health center with low neonatal surgical load.
Keywords
Tracheoesophageal fistula
Airway management
Lung isolation
INTRODUCTION
Tracheoesophageal fistula (TEF) is an anomalous connection between the trachea and esophagus with incidence of 1 in every 2500 live births.[1] TEF is classified into five types A to E according to the Gross and Vogt classification, among which the most common is Type C (80%),[2] where the upper end of the esophagus ends in a blind pouch and the lower esophagus communicates with the trachea. About 50% of cases can have associated other congenital anomalies such as Down’s syndrome and VACTERL anomaly.[3,4] The diagnosis of TEF with or without esophageal atresia requires prompt recognition of any associated conditions as surgical intervention is imminent.
CASE REPORT
A term neonate was born through lower-segment cesarean section (category II) under subarachnoid block in a peripheral center due to pregnancy-induced hypertension in the mother at 37+5 weeks gestation. The neonate (birth weight 2400 g) presented to our hospital in the emergency at 2 h of extrauterine life, with drooling of saliva and increased respiratory rate. At presentation, heart rate was 144 beats/min and respiratory rate was 64/min, along with subcostal retractions and oxygen saturation of 98% on room air. On auscultation, reduced breath sounds were heard on the left side in comparison to the right.
In view of the drooling of saliva, an attempt at the insertion of 6 French Nasogastric (NG) tube was made, but it could not be passed beyond 9 cm. An urgent chest x-ray was done [Figure 1]. In the meantime, blood samples were sent along with venous blood gas (VBG) analysis. Oxygen supplementation was given by oxygen hood. Intravenous access was secured. The chest x-ray showed coiling of the NG tube above the level of the carina. There was non-expansion of the left lung and a small gastric air bubble was seen. A possible diagnosis of TEF was made. VBG showed a pH of 7.17, partial pressure of carbon dioxide of 54 mmHg, partial pressure of oxygen of 35 mmHg, bicarbonate–16.4 meq/L, and lactates of 2.7 mmol/L. Immediate consultations were taken from the anesthesia and pediatric surgery team. The neonate’s respiratory distress continued to increase. Given the VBG findings and worsening respiratory distress, the decision for endotracheal intubation was taken. After adequate oral and nasal suctioning, pre-oxygenation was done with a neonatal self-inflating Ambu bag. Fentanyl was given at 0.5 mcg/kg to sedate the neonate. Muscle relaxant was avoided and intubation was attempted by the anesthesia team with an uncuffed endotracheal tube (ETT) of 3.5 mm. After successful intubation of the trachea, the ET tube was fixed at 9 cm after confirming bilateral air entry on auscultation. A chest x-ray was planned for confirmation of the position of ETT. Meanwhile, the neonate was shifted to the Neonatal Intensive Care Unit (NICU) and was put on mechanical ventilation on synchronized intermittent mandatory ventilation-pressure control (SIMV-PC) mode with positive inspiratory pressure of 9 cmH2O and positive end-expiratory pressure of 5 cmH2O at a fraction of inspired oxygen (FiO2) –21% as per the local intensive care unit protocols. They kept a target oxygen saturation of ≥ 94%. The neonate maintained a saturation of around 97–98% throughout the pre-operative period. The position of ETT was confirmed on chest x-ray, and a provisional diagnosis of Type C TEF with esophageal atresia was made. The position of ETT was not changed since the tip of ETT was crossing the TEF and was above the carina along with equal air entry bilaterally on auscultation. In the NICU, resuscitation and optimization were done before surgery. The surgery was planned for the next day. Frequent oral and NG suctioning was done. The neonate was kept nil per oral and was maintained on glucose infusion rate at 6 mg/kg/min.
The neonate was planned for open thoracotomy and repair of TEF. A thorough pre-operative evaluation was done to assess for the presence and severity of aspiration and hypoxemia and to rule out any underlying congenital anomalies. Pre-operative investigations showed hemoglobin of 16 g%, total leukocyte count of 13,500, and a platelet count of 39,500. Bleeding and clotting time were within normal range. Bedside echocardiography was done which showed levocardia with patent foramen ovale, mild tricuspid regurgitation, and pulmonary artery systolic pressure of 12 mmHg with good cardiac contractility and left ventricle ejection fraction of 59%. Patent ductus arteriosus of approximately 2.6 mm noted with a left to right shunt in a growing pattern was noted. Parents were counseled and a written informed consent was taken.
Thorough operation theater (OT) preparation was done on the following day. Warming was done with an underbody forced air-warming blanket and warm I.V. fluids were kept ready. Difficult airway cart was kept ready. The neonate was shifted from NICU to OT in an intubated state on bag and tube ventilation in an incubator. Standard American society of anesthesiologists (ASA) monitors, that is, neonatal pulse oximeter, neonatal blood pressure cuff (6–11 cm arm circumference), and three lead electrocardiogram were attached. A precordial stethoscope was placed on the left side of the chest. The position of ET was rechecked by auscultation, suctioning of both ETT and NG was done, and two working IV accesses were ensured (one preductal and the other postductal). Anesthesia was maintained on oxygen/air (0.5) with sevoflurane minimum alveolar concentration of 1 (MAC 1) and intermittent boluses of fentanyl (1 mcg/kg) and muscle relaxant (atracurium at 0.5 mg/kg). Ventilation was achieved on the Jackson Rees circuit with a 500 mL reservoir bag. The neonate was placed in the left lateral position with the right upper limb placed above the head to expose the right axilla. Precautions were taken not to hyperextend the shoulder joint. Adequate padding of all the pressure points (right shoulder, face, head, right arm and elbow, right hip, and both legs and feet) was done. The position of ETT was rechecked by auscultation after positioning the neonate for surgery.
Intraoperative diagnosis of type C TEF was confirmed. The fistula was ligated from the tracheal end. The non-dependent/operative lung was manually retracted by the surgeon during ligation of the azygos vein which caused periodic desaturation. A total of three episodes of desaturation were observed during the surgery, which were corrected by intermittent reinflation by manually ventilating the nondependent lung. This prevented hypoxia and corrected hypercarbia. The two esophageal ends were sutured by end-to-end anastomosis. Before esophageal anastomosis, the NG tube was manually pushed inside the esophagus to reach the stomach and in turn aid in anastomosis. The NG tube was later tapped at 20 cm at the right nostril and thus secured. Upon correcting the fistula, both lungs were manually ventilated to check for bilateral lung expansion and any leak from the fistula site. There was one episode of transient bradycardia in the neonate probably due to stimulation of the vagus nerve. It was abolished on release of traction at the surgical site. Upon successful closure of the fistula, an intercostal nerve block was given by the surgeon before closing the thoracotomy wound. The procedure was completed in 135 min. One 24G IV cannula was connected with a continuous infusion of dextrose 10% solution at 8 mL/h. Intraoperative blood loss was replaced with warm ringer lactate. No blood transfusion was done intraoperatively. A total of 35 mL of IV fluid was administered in the intraoperative period. After confirming bilateral lung ventilation, hemodynamic stability, and consultation with the surgeon, the neonate was planned to be shifted in an intubated state to NICU in an incubator. There, the neonate was maintained on SIMV-PC mode with FiO2 of 30%. Care was taken that the neonate does not pull the NG tube out so as not to disturb the anastomosis. Post-operative pain management was done with I.V. morphine infusion at 20 mcg/kg/hr. Precautions were taken in the post-operative period to safeguard the repair by keeping the head of the neonate in slight flexion using a head pillow and by avoiding any jerky movement of the neonate. A chest x-ray of the neonate in post-operative period is shown in Figure 2, depicting an endotracheal tube in situ, expansion of left lung, nasogastric tube going into the stomach and intercostal drain in situ. The post-operative course went uneventfully and the neonate was extubated on day 5 of surgery and shifted onto high flow nasal cannula. The neonate was discharged from the hospital on day 12 of life with stable vitals, on room air, and tolerating oral feeds.
DISCUSSION
According to Gross and Vogt classification, there are five types of TEF: Type A–E of which around 85% cases present with type C defect in which the upper esophageal pouch is blind and the lower esophagus communicates with the trachea.
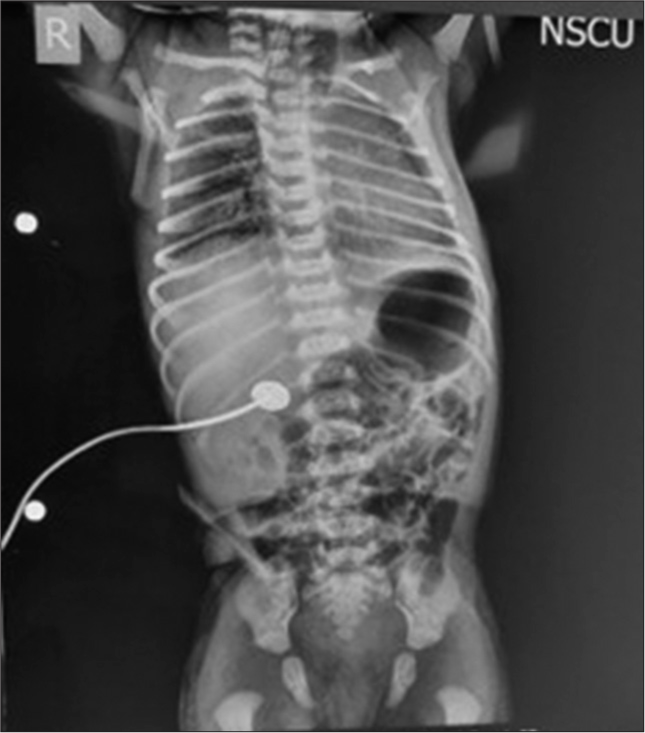
- Pre-operative chest x-ray showing coiling of nasogastric tube and non-expansion of left lung.
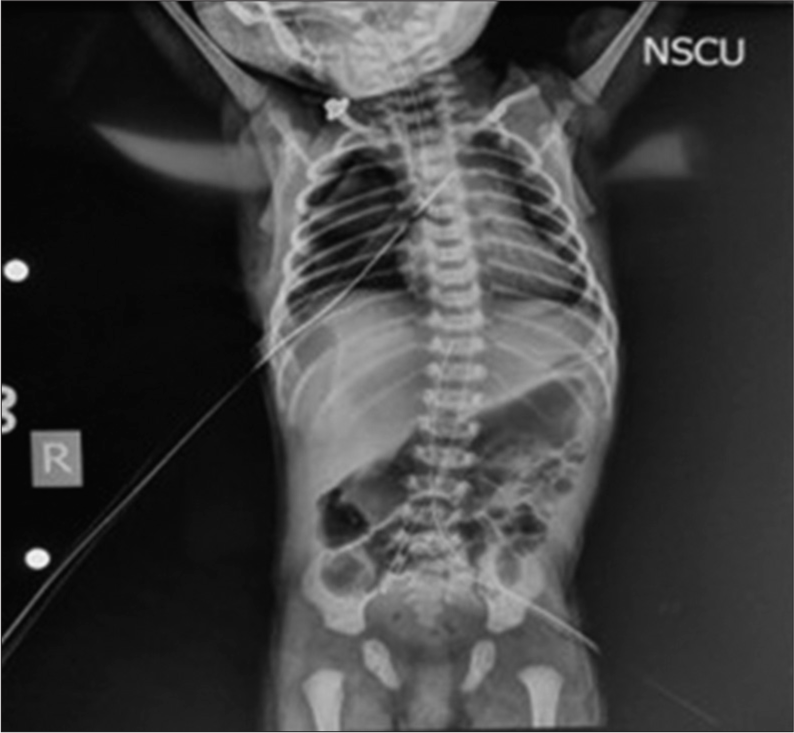
- Post-operative chest x-ray showing endotracheal tube, nasogastric going into the stomach, and intercostal drain (ICD) in situ.
TEF surgeries carry their unique challenges.
Ideal surgical conditions require lung isolation, providing the surgeon with adequate space and visualization for surgery. Equipment for neonatal one-lung ventilation includes airway devices such as different types of bronchial blockers, Marraro-bilumen tube, etc. These devices are not easily available at most centers that have low neonatal surgical caseloads. Furthermore, their optimum use requires special training. Therefore, the method most frequently used is placing the ETT tip just beyond the fistulous connection and confirmation of ETT by either fiberoptic guidance or auscultation.
Accidental extubation, tube malposition, migration, or occlusion of ETT can occur at any time during patient shifting or surgery. Late detection of these events exposes the neonate to hypoxia, hypercarbia, and acidosis which may cause reversion to fetal circulation.
Surgical complications include accidental ligation of the right main bronchus and post-operative complications such as – anastomotic leak, gastroesophageal reflux disease, dysphagia, tracheomalacia, recurrent aspiration, and esophageal stricture formation.
Sometimes, intraoperatively, a large gap between the two ends of the esophagus is found and a staged repair might be required.
Vigilant monitoring is required to ensure the smooth conduction of surgery and avoid hypoxemia, hypercarbia, hypothermia, and acidosis for optimum outcomes.
Most neonates will require post-operative care in a well-equipped NICU for the continuation of mechanical ventilation and parenteral nutrition.
Post-operative risk assessment is mandatory to timely diagnose and treat any complications after the surgery such as gastroesophageal reflux, septicemia, lung collapse, and anastomotic leak.
CONCLUSION
For good surgical outcomes in the case of TEF, closed-loop communication between the anesthesiologist, surgeon, and neonatologist is required both in the perioperative period. This case report aims to emphasize the importance of early detection and surgical intervention which has a significant impact on the morbidity and mortality of the neonate. Early detection of the fistula, quick screening for congenital anomalies before surgery, resuscitation of neonate before surgery, managing ventilatory challenges in the intraoperative period to prevent hypoxemia, hypercarbia, respiratory acidosis, reversal of flow to the fetal circulation, and prevention of hypoglycemia, hypothermia, and fluid overload leads to successful surgical outcomes.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Neonatal Factors Predictive for Respiratory and Gastro-intestinal Morbidity after Esophageal Atresia Repair. Pediatr Neonatol. 2019;60:261-9.
- [CrossRef] [PubMed] [Google Scholar]
- The Surgery of Infancy and Childhood Philadelphia, PA: WB Saunders; 1953. p. :75-102.
- [Google Scholar]
- Anesthetic Considerations for the Neonate with Tracheoesophageal Fistula. Middle East J Anaesthesiol. 2008;19:1241-54.
- [Google Scholar]






